Electrolytic Sensor Method solution|electrolytic conductivity : Brand Ions in electrolyte solutions. Electrolytic dissociation 2.2. Ion-dipole and ion-ion interactions in solutions. Interaction potentials. 2.3. Ion solvation. 2.4. . The method of a rotating disk electrode with a ring (RDER) 7.7. Comparing the rates of the stages and determining the Mechanism of the process Panobianco Vila Industrial- Campinas/Sp, Campinas. 16 likes · 9 were here. 李Melhor Academia da Região Aceitamos todos os Planos
{plog:ftitle_list}
Publicidade. Ah! No Reclame AQUI, empresas ruins, não recomendadas e em análise não são anunciadas. Caso veja alguma, não deixe de nos avisar: .
This article presents an overview and analysis of EC sensors used in these industries from the lowest to the highest range of conductivities and in applications, where there are strong requirements for accurate and traceable conductivity measurements of water and .
We studied a Mach–Zehnder Interferometer (MZI)-based electrolytic sensor on a silicon-on-insulator (SOI) platform. First, the Si waveguide, integrated with the dielectric layer (SiO2) and operating under varying pH, is designed using commercial software Nextnano. The impact of the band bending in the SiO2 integrated with the Si waveguide is presented. . Electrochemical sensor technology is an integral part of modern analytical chemistry that has attracted great attention. It is multifaceted and rapidly progressing because of its high demand and continuous technological advancements. Electrochemical sensors offer several advantages over traditional analytical methods, including selectivity, sensitivity, .Ions in electrolyte solutions. Electrolytic dissociation 2.2. Ion-dipole and ion-ion interactions in solutions. Interaction potentials. 2.3. Ion solvation. 2.4. . The method of a rotating disk electrode with a ring (RDER) 7.7. Comparing the rates of the stages and determining the Mechanism of the process
Electrolytic solutions are those that are capable of conducting an electric current. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an electrolyte is ordinary salt, sodium chloride. Solid NaCl and pure water are both non-conductive, but a solution of salt in water is readily conductive.The correct pH electrolyte will minimize potential junction errors and provide optimum temperature and time responses. The proper electrolyte to use is written on the sensor shaft. The pH electrolyte must be refilled or replaced regularly to achieve good electrode performance. Our pH sensors come with a special wetting cap that makes refilling . The pH monitoring is significantly important in chemical industry, biological process, and pollution treatment. However, it remains a great challenge to measure pH in extreme alkalinity conditions. Herein, we employ an electrolyte-gated field-effect-transistor (FET) strategy using non-stoichiometric SrCoOx with rich oxygen-vacancy defects as channel materials for . In this paper, we propose a theoretical method which utilizes a single certain sine waveform voltage to measure the electrolytic conductivity through a two-electrode conductance cell. We deduce the formula for the electrolytic solution resistance, then the electrolytic conductivity of solution can be received.
There is also a reference electrode similar to ion selective electrode, but there is no to-be-measured ion in the internal electrolyte and the selective membrane is replaced by porous frit, which allows the slow passage of the internal filling solution and forms the liquid junction with the external text solution.Simultaneously, the solution on the membrane’s left side develops a negative charge because there is an excess concentration of Cl –. We call this difference in potential across the membrane a junction potential, which we represent as E j. Figure 11.9 Origin of the junction potential between a solution of 0.1 M HCl and a solution of 0.01 M HCl. The detection range of the bio-electrolytic sensor was up to 100 mM which is much higher than that of MFC-based sensor (Kaur et al., 2013) and almost at the same level as that of the MDC-based sensor reported previously (Jin et al., 2016). It should be noted that the response time (i.e., 1 h) achieved in this study was much shorter compared to .
Electrolytic sensor method, together with dynamic relative humidity testing method and infrared testing method, belongs to sensor method water vapor permeability testing. It was gradually introduced since 1970. Requiring the same test environment as that of water method, electrolytic sensor method is distinguished for its rapid test speed, high .Electrolytic Cells. If we construct an electrochemical cell in which one electrode is copper metal immersed in a 1 M Cu 2 + solution and the other electrode is cadmium metal immersed in a \(\,1\; M\, Cd^{2+}\) solution and then close the circuit, the potential difference between the two compartments will be 0.74 V. The cadmium electrode will begin to dissolve (Cd is oxidized to . Studying the concentration and temperature dependence of the conductivity of electrolyte solution is of great significance for the evaluation and improvement of the performance of the electrochemical system. In this paper, based on the influence of the number of free ions and ion mobility on the conductivity, a semiempirical conductivity model with five .An overview and analysis of EC sensors used in these industries from the lowest to the highest range of conductivities and in applications, where there are strong requirements for accurate and traceable conductivity measurements of water and electrolyte solutions is presented. Electrolytic conductivity (EC) sensors are widely used in diverse industries, including: .
For in vitro diagnostic use. Instrument ISE 9180 IVD 9180 Electrolyte Analyzer INS_100 03157334001 ELECTROLYTE ANALYZER W/O STARTERKIT 9180 9180 Electrolyte Analyzer 04015630031832 Instruments GD5043 Not .
Production of 64 Cu via the proton irradiation of 64 Ni, electrodeposited on a suitable backing substrate, remains the most common method to produce this emerging radionuclide. Some unforeseen cases arise when the electrodeposition does not work and the electrolytic solution needs to be reprocessed, but the presence of salt buffers makes it difficult to recover the nickel . Common printing methods for sensor fabrication include screen printing 76,154,155,156, . should be below 1 V in order to prevent electrolysis of the electrolyte solution 15,115,170.For calibration, a conductance cell with a solution of known conductivity, usually potassium chloride, is employed. Calibration is rather required over the entire conductance range. Industrial conductivity probes often employ an inductive method, which has the advantage that the fluid does not wet the electrical parts of the sensor. Here, two
how to measure electrolytic error
Measurement of conductance of electrolytic solutions is done by measuring the resistance offered by the part of solution placed between the electrodes. . The other coil is a secondary coil whose one turn is in contact with the liquid and is the sensor. The induced current is the output of the sensor. . Any special type of conductivity cell .Electrolytic detection sensor method 1 Scope This part of ISO 15106 specifies an instrumental method for determining the water vapour transmission rate of . 4 Glass-fibre plate impregnated with sulfuric acid solution 5 Electrolytic cell 6 Switch valve 7 Copper tubing for carrier-gas supply (in thermostatted liquid to bring gas to test .
6 101 Helmholt Elektronik A/S, Denmark) was used to provide an additional voltage to the circuit. The 102 positive lead of the power source was connected to the anode electrode, and the negative lead was connected to a 10 Ω resistance connecting the cathode electrode103 in the circuit (Fig. 1).104 105 Fig. 1. Prototype (a) and schematic diagram (b) of the bio-electrolytic .
electrolytic sensor method has been adopted in some industrial standards (for example: medical packaging standards), there is no corresponding international support, which also impedes the popularization of industrial . this standard adds the method of using saturated saline solution to realize relative humidity. 2. Reduce the using of some . electrolytic sensor method has been adopted in some industrial standards (for example: medical packaging standards), there is no corresponding international support, which also impedes the popularization of industrial . this standard adds the method of using saturated saline solution to realize relative humidity. 2. Reduce the using of some .ISO 15106-3:2003 specifies an instrumental method for determining the water vapour transmission rate of plastic film, plastic sheeting and multi-layer structures including plastics, using an electrolytic detection sensor. NOTE The method provides rapid measurement over a wide range of water vapour transmission rates.
EDPR method is excluded due to its complexity and an alternative simple method, which does not require photolithography process. . The tested MEMS-base electrolytic tilt sensor with KOH solution shows a resolution of approximately 50 mV per 1 of inclination angle change and operating angle range of ±60 . 5. Conclusions Fig. 12.Direct current damages the sensor by causing plating of the electrodes. Our uniaxial tiltmeters use a single tilt sensor. Biaxial tiltmeters in our 500, 700 and 800-Series use two tilt sensors, oriented orthogonally. Our 900-Series biaxial clinometers use an electrolytic tilt sensor with five electrodes, arranged in the pattern of a cross. In . This sensor line provides an extra electrode (an auxiliary electrode) that can be used in compensating for baseline changes in the sensor signal. This work also offers a method for correcting an electrolytic sensor signal using temperature and humidity observations, which is useful for other, less expensive sensors, which do not have an .
how to measure electrolytic conductivity
seadoo 4 stroke compression test
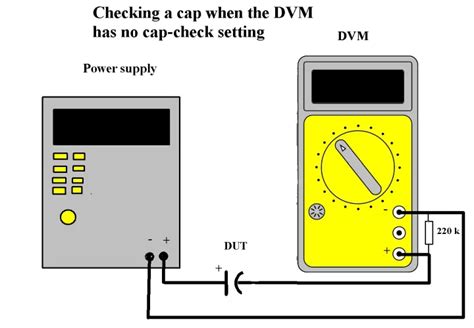
seadoo 587 compression test
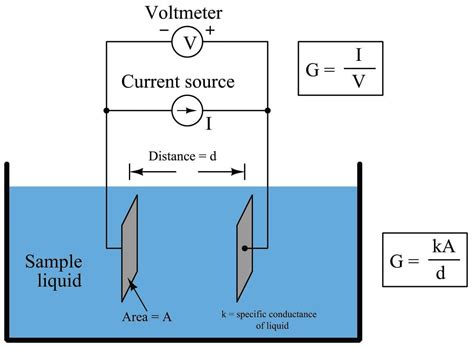
electronic conductivity sensors
webGenres Donghua. An Lin, is an orphan who lost his mother in a car accident and his father due to a gambling debt. While celebrating his 18th birthday, an old man breaks into his .
Electrolytic Sensor Method solution|electrolytic conductivity